Abstract
-
Background
- Itolizumab downregulates the synthesis of proinflammatory cytokines and adhesion molecules by inhibiting CD6 leading to lower levels of interferon-γ, interleukin-6, and tumor necrotic factor-α and reduced T-cell infiltration at inflammatory sites. This study aims to compare the effects of tocilizumab and itolizumab in the management of severe coronavirus disease 2019 (COVID-19).
-
Methods
- The study population was adults (>18 years) with severe COVID-19 pneumonia admitted to the intensive care unit receiving either tocilizumab or itolizumab during their stay. The primary outcome was clinical improvement (CI), defined as a two-point reduction on a seven-point ordinal scale in the status of the patient from initiating the drug or live discharge. The secondary outcomes were time until CI, improvement in PO2/FiO2 ratio, best PO2/FiO2 ratio, need for mechanical ventilation after administration of study drugs, time to discharge, and survival days.
-
Results
- Of the 126 patients included in the study, 92 received tocilizumab and 34 received itolizumab. CI was seen in 46.7% and 61.7% of the patients in the tocilizumab and itolizumab groups, respectively and was not statistically significant (P=0.134). The PO2/FiO2 ratio was significantly better with itolizumab compared to tocilizumab (median [interquartile range]: 315 [200–380] vs. 250 [150–350], P=0.043). The incidence of serious adverse events due to the study drugs was significantly higher with itolizumab compared to tocilizumab (14.7% vs. 3.3%, P=0.032).
-
Conclusions
- The CI with itolizumab is similar to tocilizumab. Better oxygenation can be achieved with itolizumab and it can be a substitute for tocilizumab in managing severe COVID-19.
-
Keywords: COVID-19; itolizumab; management; tocilizumab
INTRODUCTION
The coronavirus disease 2019 (COVID-19) continues to impact millions of people worldwide, prompting the science community to explore new strategies for its management. In severe COVID-19, there is a marked increase in the levels of cytokines (e.g., interleukin [IL]-6, IL-1, and IL-18 and tumor necrotic factor [TNF]) and chemokines, often referred to as a “cytokine storm” [1]. Other inflammatory markers like C-reactive protein (CRP), D-dimer, lactate dehydrogenase (LDH), and ferritin are also elevated. Hence, anti-inflammatory drugs, such as IL-6 inhibitors, IL-1 receptor antagonists, and TNF-α inhibitors have emerged as potential therapeutic agents to attenuate the release of inflammatory mediators [2].
Tocilizumab is a humanized monoclonal inhibitor of the proinflammatory cytokine IL‐6 and is licensed for use in the clinical management of cytokine release syndrome in COVID-19 [3]. Two large trials have shown that the use of tocilizumab has mortality benefits in severe COVID-19 [4,5].
Itolizumab is an anti-CD6 monoclonal antibody initially developed for various cancers but is now being repurposed for COVID-19 [6]. Itolizumab, by inhibiting CD6, downregulates the synthesis of proinflammatory cytokines and adhesion molecules, eventually leading to reduced interferon-γ, IL-6, and TNF-α and reduced T-cell infiltration at inflammatory sites [7]. Itolizumab has multiple sites of action and is expected to be better than tocilizumab in the management of COVID-19. Some studies have shown promising results with itolizumab [8,9], but no randomized controlled trial has confirmed its effectiveness. Although itolizumab has a similar mechanism of action to tocilizumab, no available study compares the two drugs in the management of COVID-19.
MATERIALS AND METHODS
Study Design and Participants
This retrospective, observational cohort study was conducted in a tertiary care hospital in India on patients with severe COVID-19 pneumonia. The study population was adults (>18 years) with severe COVID-19 pneumonia confirmed on real-time reverse transcriptase polymerase chain reaction of nasopharyngeal swabs and who had been admitted to the intensive care unit (ICU) of the center between March 2021 and June 2021.
Inclusion criteria were patients with severe COVID-19 pneumonia, defined as the clinical signs of pneumonia (fever, cough, dyspnea) plus one of the following: respiratory rate >30 breaths/min; severe respiratory distress or oxygen saturation (SpO2) <90% on room air; or PO2/FiO2 <300 at the time of admission and were treated with either tocilizumab or itolizumab during the course of their stay in the ICU. Exclusion criteria were pregnancy, death within 72 hours of therapy after excluding the adverse effects of the drugs, and patients with insufficient medical information. The exclusion criteria for using tocilizumab and itolizumab were based on the manufacturer's recommendations.
The study was approved by the Institutional Ethics Committee of All India Institute of Medical Sciences Patna, India (Ref no. AIIMS/Pat/IEC/2021/780). The need for informed consent was waived.
Data Extraction
Patients treated with tocilizumab or itolizumab were identified retrospectively by ICU records. A subsequent review of the medical records of each patient was performed to collect demographic, clinical, and laboratory information.
The following clinical information was recorded: (1) history of coexisting diseases; (2) receipt of corticosteroid, plasma therapy, or remdesivir; (3) type and duration of respiratory support; (4) PO2/FiO2 ratio; (5) adverse effects due to drugs; and (6) outcome. The following laboratory data were collected: total leukocyte count (TLC), D-dimer, ferritin, IL-6, LDH, CRP, and procalcitonin. Survival days were calculated from the day that tocilizumab/itolizumab was started to either death or discharge from the hospital. The study aimed to assess the effects of tocilizumab and itolizumab in managing severe COVID-19. The primary outcome of the study was clinical improvement (CI), defined as an at least two-point reduction on a seven-point ordinal scale in the status of patients from the time of initiating the drug or live discharge from the hospital, whichever came first.
The seven-point scale was defined as follows: 7, death; 6, hospitalisation with invasive mechanical ventilation (MV); 5, hospitalisation with non-invasive ventilation/high-flow nasal cannula; 4, hospitalisation with oxygen therapy through a non-rebreathing mask (FiO2 requirement >50%); 3, hospitalisation with oxygen therapy on low flow (FiO2 requirement 24%–50%); 2, hospitalisation not requiring oxygen therapy; 1, discharged or achieved discharge criteria (defined as clinical recovery i.e., normalization of pyrexia, respiratory rate 12–24 breaths per minute, a saturation of peripheral oxygen >94% on room air, and relief of cough, all maintained for at least 72 hours).
The secondary outcomes were (1) time until CI, (2) improvement in PO2/FiO2 ratio (defined as an increase of more than 100), (3) time to improvement in PO2/FiO2 ratio, (4) maximum PO2/FiO2 ratio, (5) need for MV after administration of study drugs, (6) duration of requirement for supplemental oxygen/non-invasive ventilation/invasive ventilation, (7) time to discharge (day of drug administration to discharge from the hospital), (8) survival days, (9) frequency of serious adverse events, and (10) mortality.
Management
All patients admitted to the ICU received standard care treatment according to the COVID-19 clinical management guidelines released by the Director-General of Health Services, Ministry of Health and Family Welfare, New Delhi, India [10]. Standard of care treatment at our institute included oxygen therapy depending on the clinical condition of the patient with a target SpO2 of 92%–96%, dexamethasone (6 mg for 10 days), and low-molecular-weight heparin for deep vein thrombosis prophylaxis. Remdesivir (200 mg IV on day 1 followed by 100 mg IV daily for 5 days) and plasma therapy were administered at the discretion of the treating clinician, according to the management guidelines [10].
In addition to standard care, IL-6 inhibitors like tocilizumab or itolizumab were given to patients with severe COVID-19 if they met the criteria for cytokine storm syndrome [11]. Tocilizumab (Actemra; Cipla Ltd.) was given at a dose of 8 mg/kg and repeated after 12–24 hours. Itolizumab (Alzumab/Alzumab-L, Biocon Biologics) was given at a dose of 1.6 mg/kg. A second dose of 0.8 mg/kg was given after 1 week if required.
Statistical Analysis
Categorical data were expressed as frequency and percentage. Continuous data were reported as the mean with standard deviation or median and interquartile range. The normality of the data was checked using the Shapiro-Wilk test and Q-Q plot. Parametric quantitative data were compared using an unpaired t‐test. Non-parametric continuous variables were compared using the Mann-Whitney U-test. Categorical data were compared with the chi‐square test or Fisher’s exact test. Multivariate logistic regression analysis was performed to assess the effects of multiple variables on the outcome. Kaplan‐Meier method and log‐rank P-value were used to compare the survival days and days to CI in the ICU. A P-value <0.05 was treated as statistically significant. Statistical analysis was conducted using SPSS software version 26 (IBM Corp.).
RESULTS
During the study period, 134 patients who were admitted to the ICU with COVID-19 received either tocilizumab or itolizumab. Of these, eight patients did not satisfy the exclusion criteria. Consequently, 127 patients were included in the study; 92 received tocilizumab and 34 received itolizumab. Most patients received two doses of tocilizumab and a single dose of itolizumab (Figure 1).
Eighty-one patients were on MV (invasive or non-invasive) at the time of administration of the study drugs, and 45 patients were on oxygen therapy. Of the 81 patients, 37 had severe acute respiratory distress syndrome (ARDS), 41 had moderate ARDS, and three had mild ARDS. which was defined as by Berlin [12].
Of the patients in our study, 76% were male and the median age was 62 years. At the time of drug administration, the median PO2/FiO2 ratio was 110 and 33.3% of the patients had severe ARDS. There was no significant difference between age, sex, comorbidities, baseline PO2/FiO2, and MV at the time of administration of drugs in the two groups (Table 1). The use of dexamethasone, remdesivir, and plasma therapy was also comparable in the two groups (Table 1).
CI was seen in 46.7% and 61.7% of the patients in the tocilizumab and itolizumab groups, respectively, differences there were not significant (P=0.134). The time to CI was also non-significantly different between the tocilizumab and itolizumab groups (median [interquartile range]: 12 days [9–16] vs. 11 days [7–12.5], P=0.253) (Table 2). In our study, all-cause mortality at 28 days was significantly lower in those receiving itolizumab compared to the tocilizumab group (32.3 vs. 54.3%, P=0.028). All-cause mortality at 60 days was lower with itolizumab compared to tocilizumab (38.2% vs. 56.5%), but the difference was not significant (P=0.068).
The patients were stratified based on IL-6 levels. A total of 86 patients with baseline IL-6 levels >30 pg/ml was placed into the high-level group. The CI in patients with higher IL-6 levels was significantly higher with itolizumab compared to tocilizumab (67% vs. 43%, P=0.035). Mortality at 28 days in patients with a higher level of IL-6 was also significantly lower with itolizumab compared to tocilizumab (27% vs. 55%, P=0.011) (Table 3)
The improvement in the PO2/FiO2 ratio after treatment was not significant between groups. However, the number of days required to achieve an improvement of 100 units in the PO2/FiO2 ratio was significantly less with itolizumab compared to tocilizumab (6 days [4–8] vs. 8 days [6–12], P=0.028). The best PO2/FiO2 ratio achieved was also significantly better with itolizumab compared to tocilizumab (315 [200–380] vs. 250 [150–350], P=0.043). The numbers of patients needing MV and weaned from it after administration of study drugs were comparable between the groups.
Duration of patients on MV, oxygen therapy, and time to discharge were also comparable in the two groups (Table 2). The incidence of serious adverse events due to the study drugs was significantly higher with itolizumab compared to tocilizumab (14.7% vs. 3.26%, P=0.032). In the tocilizumab group, two patients had a high-grade fever and one had liver dysfunction after therapy. In the itolizumab group, two patients had bronchospasm, two patients had a high-grade fever, and one had arrhythmias. There were no deaths due to adverse events in either of the groups. Extrapulmonary complications, such as acute kidney injury and sepsis, were comparable in the two groups (Table 2).
Multivariate logistic regression was carried out to assess the effects of factors on the likelihood of CI. The following factors were considered in the multivariate analysis: age; sex; comorbidities of hypertension, diabetes, chronic kidney disease, coronary artery disease, or hypothyroidism; MV at baseline; study drugs; treatment with remdesivir; plasma therapy; PO2/FiO2 ratio at baseline; and baseline laboratory results of like TLC, ferritin, LDH, CRP, D-dimer, IL-6, and procalcitonin. The overall model was significant compared to the null model (χ2 (24)=78.64, P<0.001) and explained 62.2% of the variation of CI (Nagelkerke R2). The independent factors significantly affecting CI were comorbidities, MV before administration of the study drugs, and baseline PO2/FiO2 ratio (Table 4). The odds of CI in patients without comorbidities were 24.66 times the odds of CI in patients with comorbidities. The odds of CI in patients on oxygen therapy were 25.90 times the odds of CI in patients on MV before study drug administration. There was no significant difference among the baseline parameters (comorbidities, baseline MV, and baseline PO2/FiO2 ratio) that significantly affected the CI between the two groups (Table 1).
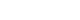
Kaplan-Meier survival plots for the two drugs to estimate CI and death at 60 days were plotted (Figures 2 and 3). The estimated median time for CI was 12 days and 11 days in the tocilizumab and itolizumab groups, respectively, which was not significantly different (log-rank P=0.080) (Figure 2). The estimated median time for death was 13 days and 14 days in the tocilizumab and itolizumab groups, respectively, and the difference was not significant (log-rank P=0.089) (Figure 3).
DISCUSSION
Initial trials on tocilizumab failed to show any mortality benefit [13-15]. These results could be attributed to small sample sizes, exclusion of critically ill patients [14,15], and imbalances in steroid use. The Randomized Evaluation of COVID-19 Therapy (RECOVERY) trial was the largest involving tocilizumab, with 4,116 adults, and revealed a significant mortality benefit with the use of tocilizumab over usual care (31% vs. 35%) [5]. The Randomised Embedded Multi-factorial Adaptive Platform Trial for Community Acquired Pneumonia trial also concluded that, in critically ill COVID-19 patients, treatment with the IL-6 receptor antagonists tocilizumab and sarilumab improved outcomes, including survival [4]. The World Health Organisation recently added tocilizumab to the list of prequalified treatments for COVID-19 [16]. In India, itolizumab was approved for “restricted emergency use” to treat COVID-19 patients [17]. However, the evidence in favor is not conclusive due to the lack of trials and small sample sizes.
A phase II trial in which 20 patients received itolizumab and 10 were controls showed 100% recovery in the treated arm versus 70% in the control arm. There was also a significant improvement in key efficacy parameters of lung function, such as PaO2 and SpO2, without increasing oxygen flow in the itolizumab arm [17]. Two studies evaluating the role of itolizumab on COVID-19 are available on preprint servers. One study shows that a single dose of itolizumab decreased the serum IL-6 levels after 48 hours of administration in 24 moderate to critically ill elderly patients with COVID-19 [18]. Another study concluded that, in 19 moderately ill elderly patients with COVID-19, itolizumab was associated with a significantly reduced risk of admission to the ICU and a 10 times lower risk of death [8]. However, despite positive results in these trials, there is no randomized clinical trial on itolizumab.
The results showed no statistical difference in CI between the two groups, which was the primary outcome of the study. However, the maximum PO2/FiO2 ratio achieved after using the study drugs was significantly higher with itolizumab compared to tocilizumab. The number of days needed to improve PO2/FiO2 ratio was significantly fewer in the itolizumab group. The all-cause mortality at 28 days was significantly lower in itolizumab compared to tocilizumab (32.3% vs. 54.3%). However, the difference in all-cause mortality at 60 days was not significantly different (38.2% vs. 56.5%). This might be due to dilution of the potential effects of the study drugs and confounding factors during prolonged ICU stay. The patients with higher IL-6 values (>30 pg/ml) showed significantly better CI and lower mortality with itolizumab compared to tocilizumab. This signifies that patients with higher IL-6 values responded better to itolizumab.
The results of the RECOVERY trial showed a 28-day mortality of 31% in the tocilizumab group. The higher mortality in our study might be due to a greater percentage of patients on MV before the start of treatment and a longer assessment time of 60 days. In our study, 67% of patients in the tocilizumab group were on MV, whereas 54% of those in the RECOVERY trial were on MV. A case series on 20 patients treated with itolizumab showed a mortality rate of 35% in patients with moderate COVID-19 ARDS compared to 60% in those who had not been treated with an IL-6 inhibitor [10]. In one study, only one death occurred in 19 elderly patients with moderate COVID-19 treated with itolizumab [9]. The results of these two cohorts cannot be compared due to differences in baseline characteristics, study design, management protocols, and quality of care. As severe COVID-19 has a longer course, we followed patients for a longer duration to reduce exclusions.
The median time to discharge was 20 days in both groups, similar to the findings of the RECOVERY trial. The number of patients weaned from MV in our study was about 17% in both groups. The RECOVERY trial showed similar proportions of patients weaned from MV in the tocilizumab and control group (35% vs. 31%). As our study did not include a control group, it is difficult to conclude whether IL-6 inhibitors benefit patients who are already on invasive MV at the time of drug administration.
The time needed to improve the PO2/FiO2 ratio was significantly shorter with itolizumab than with tocilizumab (6 vs. 8 days). The best PO2/FiO2 ratio achieved after therapy was also significantly better with itolizumab. No large trial on tocilizumab (REMAP-CAP or RECOVERY) has evaluated the effect on the PO2/FiO2 ratio.
The incidence of serious adverse effects was significantly higher with itolizumab. Two patients had severe bronchospasm and desaturation after the administration of itolizumab. A high number of adverse events occurred with itolizumab, even after premedication with hydrocortisone and pheniramine. Both cases of bronchospasm occurred with the lyophilized form of itolizumab.
Multivariate logistic regression analysis was performed to determine the confounding variables affecting our primary outcome. We found comorbidities, baseline MV, and baseline PO2/FiO2 ratio as the factors affecting the CI. However, these confounding factors were distributed equally between the two groups. There was no significant difference in the survival plots for CI or mortality between the two groups. The RECOVERY trial has found significant mortality benefits of 4% with tocilizumab compared to the control group (31% vs. 35%). We found better CI and survival rates with itolizumab, with a difference of 15% and 18%, respectively, compared to tocilizumab. Although the difference was large, it did not reach a significant level due to the small sample size. However, this difference is clinically significant in routine practice.
Our study has some limitations. First, it was a retrospective single-center study. Second, it was not a randomized comparison, so unmeasured confounding variables cannot be ruled out. Third, the use of the study drugs depended on the diagnosis of cytokine storm syndrome at the discretion of the clinician with no objective criteria. Thus, the baseline parameters might not match between the two groups. Fourth, the sample size of our study was small. The itolizumab group had fewer patients because the drug recently was approved for use, and the clinicians lacked experience in using it compared to tocilizumab. Fifth, we did not include a control group in our study. Sixth, it is difficult to consider all adverse effects of the drugs due to the numerous factors affecting the clinical features of a critically ill patient. This might have led to less reporting of adverse drug effects. In addition, in the multivariate analysis, many variables were adjusted, which might have led to the overfitting of the model.
Our study also has strengths. First, it is the first study to compare itolizumab with tocilizumab in the treatment of COVID-19. Second, the follow-up period was long, and we calculated 60-day mortality considering the longer stay of patients with COVID-19 in the ICU. Third, we collected data on daily changes in the PO2/FiO2 ratio, demonstrating a significant secondary outcome.
The CI with itolizumab is similar to that of tocilizumab. Itolizumab had a 28-day mortality benefit over tocilizumab but did not at 60 days. Better oxygenation can be achieved with itolizumab, which can be a substitute for tocilizumab in managing severe COVID-19. Large randomized controlled trials to compare the two drugs are needed.
KEY MESSAGES
▪ Itolizumab, an interleukin-6 inhibitor, has multiple sites of action and is expected to be better in the management of coronavirus disease 2019 (COVID-19) than tocilizumab.
▪ The clinical improvement and survival rates with itolizumab are similar to those of tocilizumab; however, tocilizumab has a better safety profile compared to itolizumab.
▪ Better oxygenation can be achieved with itolizumab and it can be a substitute for tocilizumab in managing severe COVID-19.
NOTES
-
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
-
FUNDING
None.
-
ACKNOWLEDGMENTS
The study was presented at the 42nd International Symposium on Intensive Care & Emergency Medicine in Brussels, Belgium, which was held from March 21 to 24, 2023.
-
AUTHOR CONTRIBUTIONS
Conceptualization: AK (Abhyuday Kumar), NK. Data curation: AK (Abhyuday Kumar), NK, AP. Formal analysis: AK (Ajeet Kumar), SP, PMN. Methodology: AK (Abhyuday Kumar), NK, AK (Ajeet Kumar). Project administration: AK (Ajeet Kumar), SP, PMN. Visualization: AK (Ajeet Kumar), NK. Writing–original draft: AK (Ajeet Kumar), NK, RD. Writing–review & editing: AP, RD.
Figure 1.Study cohort.

Figure 2.Kaplan-Meier estimate of the cumulative probability of clinical improvement in the treatment group.
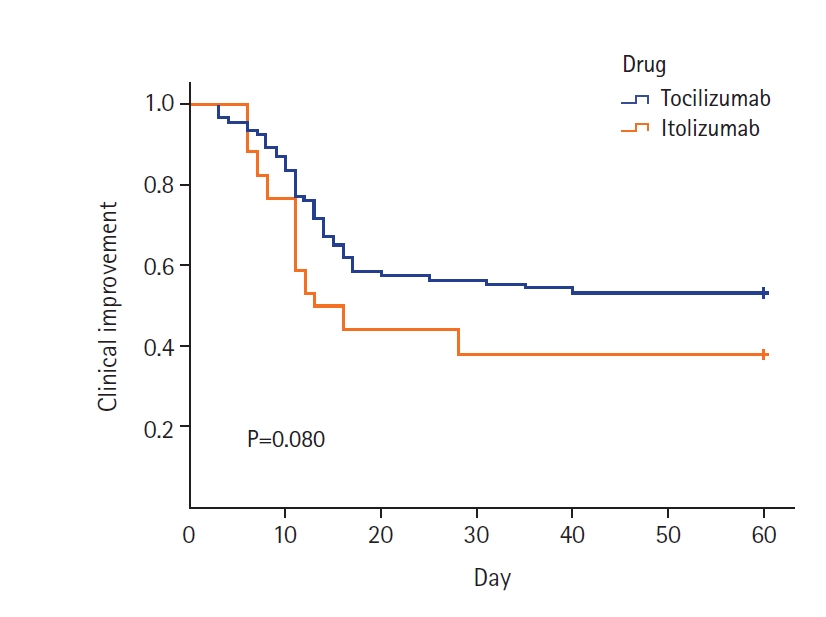
Figure 3.Kaplan-Meier estimate of the cumulative probability of mortality by treatment group.
Table 1.Comparison of demographics and baseline characteristics of the patients
|
Baseline characteristics |
Tocilizumab (n= 92) |
Itolizumab (n= 34) |
P-value |
|
Age (yr) |
62 (53–70) |
62 (50–64) |
0.466 |
|
Sex (male:female) |
70:22 |
26:8 |
0.170 |
|
Any comorbidity |
73 (79) |
26 (76) |
0.727 |
|
Diabetes |
48 (52) |
19 (56) |
0.711 |
|
Hypertension |
48 (52) |
17 (50) |
0.828 |
|
Chronic renal insufficiency |
4 (4) |
2 (3) |
0.661 |
|
Hypothyroidism |
9 (10) |
2 (3) |
0.726 |
|
Cardiovascular disease |
4 (4) |
3 (9) |
0.386 |
|
MV at study drug administration (invasive or non-invasive) |
62 (67) |
19 (56) |
0.171 |
|
Baseline PO2/FiO2 ratio at study drug administration |
100 (81–150) |
120 (100–152) |
0.268 |
|
Time to infusion of study drug after admission (day) |
5 (3–7) |
4 (3–6) |
0.312 |
|
Use of corticosteroids |
83 (90) |
30 (88) |
0.745 |
|
Use of remdesivir |
86 (93) |
32 (94) |
0.896 |
|
Use of plasma therapy |
67 (73) |
26 (76) |
0.680 |
|
TLC (1,000/µl) |
12 (9–14.9) |
14 (10.8–17.2) |
0.121 |
|
D-dimer (µg/ml) |
1.15 (0.72–2.55) |
1.01 (0.7–1.91) |
0.594 |
|
Ferritin (ng/ml) |
735 (379–958) |
411 (331–979) |
0.466 |
|
IL-6 (pg/ml) |
55 (23–175) |
82 (44–219) |
0.066 |
|
LDH (U/L) |
1,076 (856–1,349) |
1,100 (760–1,329) |
0.781 |
|
CRP (mg/l) |
98 (56–153) |
106 (51.5–127.5) |
0.644 |
|
Procalcitonin (ng/ml) |
0.82 (0.60–1.30) |
0.80 (0.45–1.20) |
0.335 |
Table 2.Comparison of the outcome variables in tocilizumab and itolizumab groups
|
Variable |
Tocilizumab (n=92) |
Itolizumab (n=34) |
P-value |
|
Clinical improvement |
43 (46.7) |
21 (61.7) |
0.134 |
|
Day of clinical improvement |
12 (9–16) |
11 (7–13) |
0.253 |
|
Mortality (28 days) |
50 (54.3) |
11 (32.3) |
0.028 |
|
Mortality (60 days) |
52 (56.5) |
13 (38.2) |
0.068 |
|
PO2/FiO2 ratio improvement |
50 (54.3) |
23 (67.7) |
0.180 |
|
Day of PO2/FiO2 ratio improvement |
8 (6–12) |
6 (4–8) |
0.028 |
|
Maximum PO2/FiO2 ratio |
250 (150–350) |
315 (200–380) |
0.043 |
|
Need for MV after therapy |
12 (13.0) |
4 (11.7) |
0.848 |
|
Weaned from MV after therapy |
16 (17.4) |
6 (17.6) |
0.973 |
|
Patients on MV during the stay |
73 (79.3) |
23 (67.6) |
0.171 |
|
MV days |
10 (5–13) |
8 (7–10) |
0.821 |
|
Oxygen therapy days |
11 (4–16) |
10 (5–14) |
0.787 |
|
Time to discharge |
20 (16–26) |
20 (15–23) |
0.375 |
|
Serious adverse event |
3 (3.3)a)
|
5 (14.7)b)
|
0.032 |
|
AKI |
5 (5.4) |
2 (5.9) |
1.000 |
|
Sepsis |
12 (13.0) |
4 (12.5) |
1.000 |
Table 3.Clinical Improvement and 28-day mortality in patients with high IL-6 (>30 pg/ml)
|
Variable |
Tocilizumab (n=56) |
Itolizumab (n=30) |
P-value |
|
Clinical improvement |
24 (43) |
20 (67) |
0.035 |
|
28-Day mortality |
31 (55) |
8 (27) |
0.011 |
Table 4.Multivariate logistic regression analysis of factors affecting clinical improvement
|
Variable |
OR (95% CI) |
P-value |
|
Age (yr) |
1.12 (1.00–1.26) |
0.343 |
|
Sex |
|
0.893 |
|
Male |
0.86 (0.21–3.48) |
|
|
Female |
Reference |
|
|
Comorbidity |
|
0.009 |
|
Present |
Reference |
|
|
Absent |
24.66 (2.19–277.06) |
|
|
Hypertension |
|
0.514 |
|
Present |
Reference |
|
|
Absent |
1.61 (0.38–6.81) |
|
|
Diabetes |
|
0.110 |
|
Present |
Reference |
|
|
Absent |
3.5 (0.21–4.66) |
|
|
CKD |
|
0.618 |
|
Present |
Reference |
|
|
Absent |
2.12 (0.04–18.93) |
|
|
CAD |
|
0.122 |
|
Present |
Reference |
|
|
Absent |
7.91 (0.57–108.81) |
|
|
Hypothyroidism |
|
0.576 |
|
Present |
Reference |
|
|
Absent |
0.48 (0.03–6.21) |
|
|
MV at baseline |
|
0.001 |
|
Present |
Reference |
|
|
Absent |
25.90 (4.99–134.30) |
|
|
Study drug |
|
0.707 |
|
Tocilizumab |
Reference |
|
|
Itolizumab |
1.3 (0.32–5.2) |
|
|
Remdesivir |
2.85 (0.05–15.9) |
0.428 |
|
Plasma therapy |
0.61 (0.21–4.5) |
0.532 |
|
PO2/FiO2 ratio at baseline |
0.97 (0.94–0.99) |
0.023 |
|
TLC |
1.06 (0.97–1.17) |
0.183 |
|
Ferritin |
1.00 (1.00–1.00) |
0.970 |
|
LDH |
1.00 (1.00–1.00) |
0.777 |
|
CRP |
0.99 (0.99–1.00) |
0.791 |
|
D-dimer |
1.12 (0.80–1.55) |
0.500 |
|
IL-6 |
1.00 (1.00–1.00) |
0.387 |
|
Procalcitonin |
1.55 (0.69–3.46) |
0.282 |
References
- 1. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet 2020;395:1033-4.ArticlePubMedPMC
- 2. Heimfarth L, Serafini MR, Martins-Filho PR, Quintans JS, Quintans-Júnior LJ. Drug repurposing and cytokine management in response to COVID-19: a review. Int Immunopharmacol 2020;88:106947. ArticlePubMedPMC
- 3. U.S. Food and Drug Administration. Coronavirus (COVID-19) update: FDA authorizes drug for treatment of COVID-19 [Internet]. U.S. Food and Drug Administration. 2021;[cited 2024 Jan 20] Available from: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-drug-treatment-covid-19.
- 4. REMAP-CAP Investigators; Gordon AC, Mouncey PR, Al-Beidh F, Rowan KM, Nichol AD, et al. Interleukin-6 receptor antagonists in critically ill patients with COVID-19. N Engl J Med 2021;384:1491-502.ArticlePubMed
- 5. RECOVERY Collaborative Group. Tocilizumab in patients admitted to hospital with COVID-19 (RECOVERY): a randomised, controlled, open-label, platform trial. Lancet 2021;397:1637-45.PubMedPMC
- 6. Ministry of Health and Family Welfare. DCGI gives nod for restricted emergency use to itolizumab for moderate to severe COVID-19 patients [Internet]. Ministry of Health and Family Welfare. 2020;[cited 2024 Jan 20] Available from: https://pib.gov.in/PressReleasePage.aspx?PRID=1637926.
- 7. Loganathan S, Athalye SN, Joshi SR. Itolizumab, an anti-CD6 monoclonal antibody, as a potential treatment for COVID-19 complications. Expert Opin Biol Ther 2020;20:1025-31.ArticlePubMed
- 8. Díaz Y, Ramos-Suzarte M, Martín Y, Calderón NA, Santiago W, Viñet O, et al. Use of a humanized anti-CD6 monoclonal antibody (itolizumab) in elderly patients with moderate COVID-19. medRxiv [Preprint]. 2020 Jul 24 [cited 2024 Jan 20]. Available from: https://doi.org/10.1101/2020.07.24.20153833.Article
- 9. Kumari P, Kumar A, Sinha C, Kumar A, Singh PK, Arun SK. Off-label use of itolizumab in patients with COVID-19 ARDS: our clinical experience in a dedicated COVID center. Indian J Crit Care Med 2021;25:467-9.ArticlePubMedPMC
- 10. Directorate General of Health Services; Ministry of Health and Family Welfare; Government of India. Clinical management protocol: COVID-19 [Internet]. Ministry of Health and Family Welfare, Government of India. 2020;[cited 2024 Jan 20]. Available from: https://www.mohfw.gov.in/pdf/ClinicalManagementProtocolforCOVID19.pdf.
- 11. Lee DW, Gardner R, Porter DL, Louis CU, Ahmed N, Jensen M, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood 2014;124:188-95.ArticlePubMedPMCPDF
- 12. Fanelli V, Vlachou A, Ghannadian S, Simonetti U, Slutsky AS, Zhang H. Acute respiratory distress syndrome: new definition, current and future therapeutic options. J Thorac Dis 2013;5:326-34.PubMedPMC
- 13. Stone JH, Frigault MJ, Serling-Boyd NJ, Fernandes AD, Harvey L, Foulkes AS, et al. Efficacy of tocilizumab in patients hospitalized with COVID-19. N Engl J Med 2020;383:2333-44.ArticlePubMed
- 14. Hermine O, Mariette X, Tharaux PL, Resche-Rigon M, Porcher R, Ravaud P, et al. Effect of tocilizumab vs usual care in adults hospitalized with COVID-19 and moderate or severe pneumonia: a randomized clinical trial. JAMA Intern Med 2021;181:32-40.PubMed
- 15. Salvarani C, Dolci G, Massari M, Merlo DF, Cavuto S, Savoldi L, et al. Effect of tocilizumab vs standard care on clinical worsening in patients hospitalized with COVID-19 pneumonia: a randomized clinical trial. JAMA Intern Med 2021;181:24-31.ArticlePubMed
- 16. World Health Organisation. WHO prequalifies first monoclonal antibody - tocilizumab - to treat COVID-19 [Internet]. World Health Organisation. 2022;[cited 2024 Jan 20]. Available from: https://www.who.int/news/item/11-02-2022-who-prequalifies-first-monoclonal-antibody---tocilizumab-to-treat-covid-19.
- 17. Biocon.com. Biocon presented insights into clinical study that enabled DCGI approval of itolizumab for COVID19 [Internet]. Biocon.com. 2022;[cited 2024 Jan 20]. Available from: https://www.biocon.com/biocon-presented-insights-into-clinical-study-that-enabled-dcgi-approval-of-itolizumab-for-covid19.
- 18. Saavedra D, Añé-Kourí AL, Sánchez N, Filgueira LM, Betancourt J, Herrera C, et al. An anti-CD6 monoclonal antibody (Itolizumab) reduces circulating IL-6 in severe COVID-19 elderly patients. Research Square [Preprint]. 2020 [cited 2024 Jan 20]. Available from: https://doi.org/10.21203/rs.3.rs-32335/v1.Article
Citations
Citations to this article as recorded by

 , Neeraj Kumar1
, Neeraj Kumar1 , Arunima Pattanayak2
, Arunima Pattanayak2 , Ajeet Kumar1
, Ajeet Kumar1 , Saravanan Palavesam1
, Saravanan Palavesam1 , Pradhan Manigowdanahundi Nagaraju1
, Pradhan Manigowdanahundi Nagaraju1 , Rekha Das3
, Rekha Das3






 KSCCM
KSCCM


 PubReader
PubReader ePub Link
ePub Link Cite
Cite